Hey guys, today I will be doing an overview for Endothermic reactions.
An Endothermic reaction is when there is energy taken in from the surroundings. This can be shown by a decrease in temperature on a thermometer.
Bond Breaking = ENDOTHERMIC
This is important as when a product is formed there is going to be energy release. But in an ENDOTHERMIC reaction, the energy in bond making is more than the energy for bond breaking (which is the reactants). Resulting in this positive energy change.
One example would be: Photosynthesis. –> As the Light energy from the sun is TAKEN IN from the surroundings for the reaction to occur.
Another example would be: Thermal Decomposition
The reaction for Thermal Decomposition is:
Calcium Carbonate (+ heat) –> Carbon Dioxide + Calcium Oxide
Endothermic reactions do have a role in daily life for us too! Such as: Ice packs or Sports injury packs. As, the chemical reaction allows the pack to become suddenly cooler without having to put it in the freezer.
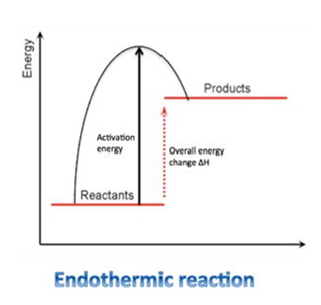
This figure is a reaction profile for ENDOTHERMIC reactions.
As you can see the Products have more energy than the reactants. The difference in the height would represent the net energy change during the reaction. This is also known to be a positive(+) enthalpy change.
The Activation Energy is the minimum amount of energy required to start off a reaction.
The Activation Energy can be lowered by having a catalyst. Now a catalyst simply helps to speed up the rate of reaction and so makes it faster.
The catalyst can lower the activation energy by providing an alternative way to start off the reaction. Therefore, speeding up the rate of reaction.
Please if you have any further questions, feel free to contact me!

By Anshjeet Singh
Subscribe to My Newsletter for weekly articles all for FREE!

2 Responses
Very informative. Thanks
Thanks!